How can the REACH revision secure future-proof products for Europe?
The EU is about to start reforming its flagship chemicals legislation, REACH, making it a pivotal moment for anyone working in the chemical industry. Yet the anticipated changes to REACH as announced in the European Commission’s Chemicals Strategy for Sustainability will not only affect chemicals production but are very likely to have a significant impact on all value chains relying in chemicals.
At a recent Digital Dialogue, Cefic gathered a large group of policy makers, chemical and downstream industry representatives to try to understand what an efficient and effective revision of REACH could look like and discuss remaining issues and concerns.
REACH will set the agenda for EU chemicals management for decades to come – we need to “get it right”
The European Green Deal goes ahead, as do all of its policies, raised Patrick Child, Deputy Director General at DG Environment, European Commission as he set the scene. The Commission intends to maintain the high ambition on this file and is committed to delivering on the REACH and CLP revision under its current term. The Commission has heard the industry and agrees with the importance of a Transition Pathway to enable the chemicals sector to adapt and yet continue to thrive.
“We support the transition to green and sustainable chemicals production, which we believe will not only benefit European society and European markets but will also help us show that the chemicals made in Europe are synonymous with safe and sustainable chemicals”
Patrick Child, Deputy Director General, DG Environment, European Commission
Thomas Casparie, Senior Vice President Chemicals, Shell reiterated the industry’s support to the goals of the Chemicals Strategy for Sustainability.
“We fully understand the desire to go at speed but it is important to get REACH right because it really sets the agenda for the industry for the next decades. In this regard, creating a stable framework that gives the industry predictability to plan investments into innovation and ensuring coherence with other pieces of legislation will be crucial”.
Thomas Casparie, Senior Vice President Chemicals, Shell
After setting the scene, the Dialogue moved to an in-depth discussion about the most important aspects of the upcoming revision.
Successful registration of polymers will depend on clear criteria
Unlike all traditional chemicals substances (monomers), polymers are currently exempt from the REACH registration. The upcoming REACH revision is likely to change this. The key question is how to make registration of polymers workable : according to some estimates, there may be between 70,000 and 400,000 polymers on the market. Distinguishing between those that pose concern and require registration and further regulatory management from the low-concern ones will therefore be key for the successful implementation of this new policy measure.
Lina Dunauskiené, Policy Officer, DG GROW, European Commission presented the Commission’s approach to grouping polymers for registration based on “Polymers Requiring Registration”: using a set of criteria to select polymers that may present some hazards.
Heli Hollnagel, a regulatory toxicologist at Dow and member of the Polymer Issue Team at Cefic agreed with the Commission that designing good criteria to allow screening of polymers with higher likelihood of hazard is the main challenge. Considering the sheer number of polymers, there should be a “mindset shift” from focusing on maximum data collection for all substances to prioritising those that pose the biggest concern.
Registration of polymers will impact a large number of downstream industries that use polymers in their chemicals mixtures, like the detergents, cleaning and maintenance product sector. Many of them will face registration obligations for the first time. This particular sector still remains concerned as no clear rules on grouping have been circulated yet, making it impossible for the industry to prepare, according to Jan Robinson, Scientific and Regulatory Affairs Director, International Association for Soaps, Detergents and Maintenance Products (AISE). As a result, the actual number of registrations and associated costs may turn out to be greater than anticipated.
Registration of polymers does not only mean additional costs for the industry, it also raises an important question of the testing methods to check the safety of these substances. There is a high risk of conducting millions of redundant animal tests while registering polymers. To avoid this, it is paramount to have clear registration criteria, develop new and validate existing non-animal tests to assess polymer bioavailability, argued Emily McIvor, science policy advisor at PETA Science Consortium International
“The animal protection community from the start has been extremely worried about the impact of potential registration on animal testing. In particular, there is a high risk of redundant testing and tests being used that are not validated for the purpose intended and leading to questionable regulatory impact”
Emily McIvor, Science Policy Advisor, PETA Science Consortium International
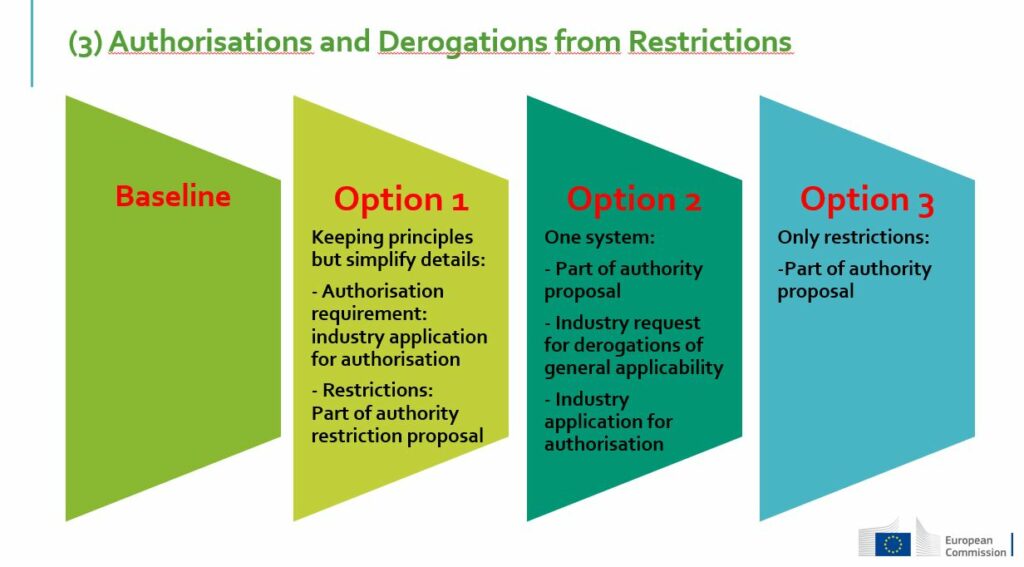
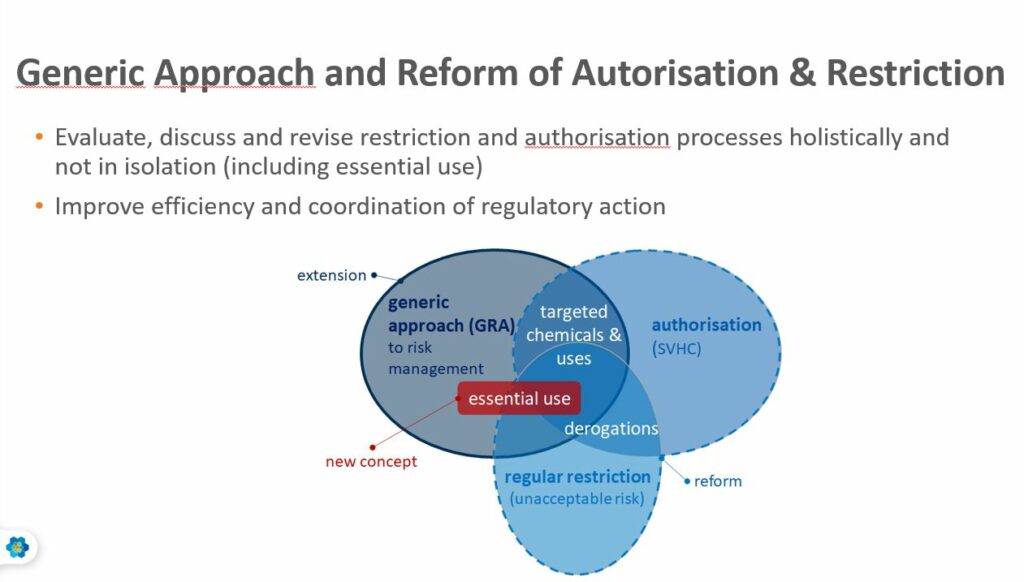
REACH Authorisation & Restriction reform should simplify and accelerate decision making on chemicals
Otto Linher, explained that the Commission had been too slow to regulate certain substances in the past, especially with endocrine disruptors and persistent substances, and has faced a long backlog of authorisation and restriction requests.
“I think we need to realise that some of the criteria that we’ve used in the past led us to getting lost in detail. What I mean here is that we’ve been overly focusing on the burden of proof for the last detail of analysis and we lost the broad picture and what the real questions underlying those issues were” .
Otto Linher, Senior Expert, REACH Unit, European Commission
Otto Lihner described four steps to deal with authorisation and restriction:
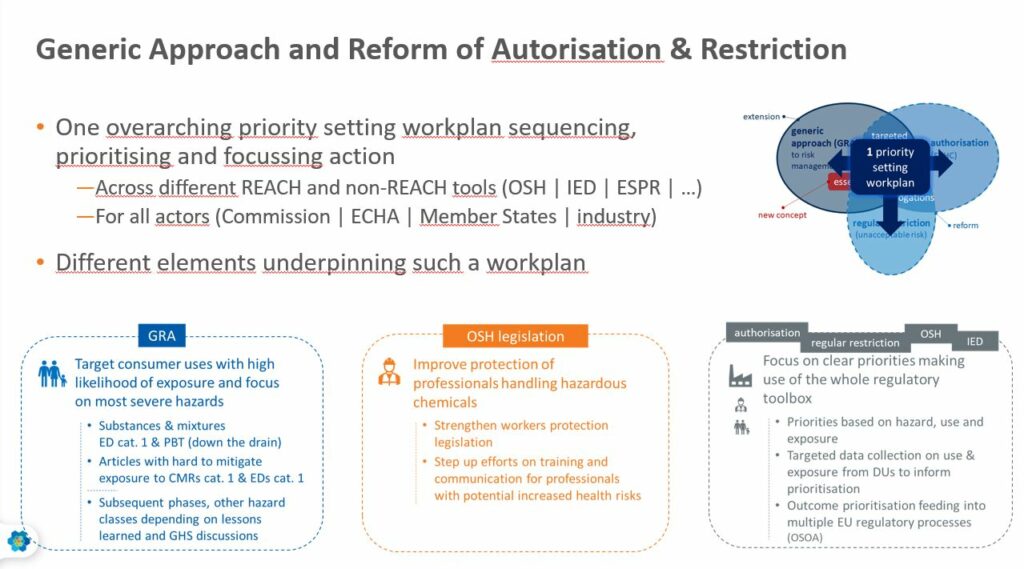
1 – Prioritisation of substances for regulatory action using the PACT tool and Candidate Listing. The Commission wants to add a requirement to provide more information about uses during the Candidate listing, as having this information earlier in the process will speed up regulatory action. The Candidate List will not only be used as the first step authorisation but also for restrictions and for other regulatory measures, including those falling under Occupational Safety and Health (OSH) and Industrial Emissions Directive (IED).
2 – Restricting substance use (68(1), 68(2), Title VII, 69(2)). This would be extended to new hazard classes, in particular endocrine disruptor, persistent substances, specific target organ toxicity (STOT) and respiratory sensitizers and certain professional uses in order to have a faster way to restrict without the need to prove on a case by case basis.
3 – Dealing with the need for continued use: processes for granting authorisation and derogations
4 – Criteria to assess justification of authorisations and derogations including a new derogation for essential uses, which is also expected to speed up decision making (no detailed assessment will be needed for substances that will be deemed non-essential for health, safety or functioning of society).
“We need to avoid the risk of putting in place solutions that might look ideal for the generic approach or for authorisation or restriction if you look at them in isolation. But when you put them all together, these solutions may appear less optimal”. Steven van de Broeck, Director, REACH and chemicals policy, Cefic.
Steven van de Broeck, Director REACH and Chemicals Policy, Cefic.
Downstream users remain concerned about impact of revision on their sectors
Revision of REACH will have an impact not only on the chemical industry but all sectors along the supply chain that rely on chemicals to manufacture their final products. Didier Leroy, Technical and Regulatory Affairs Director of the European Council of the Paint, Printing Ink, and Artist’s Colours Industry (CEPE), warned about the risk that many substances may disappear and the proposed hazard-based approach to chemicals management means “moving away from science”. Coating and paints has been identified as one of the most impacted industry sectors in the Economic Analysis of the business impacts of the Chemicals Strategy for Sustainability commissioned by Cefic.
Ignacio Doreste, Senior Advisor at the European Trade Union Confederation (ETUC), stressed that there should be more synergies between REACH and EU Occupational Health and Safety (OHS) legislation to avoid duplication of work.
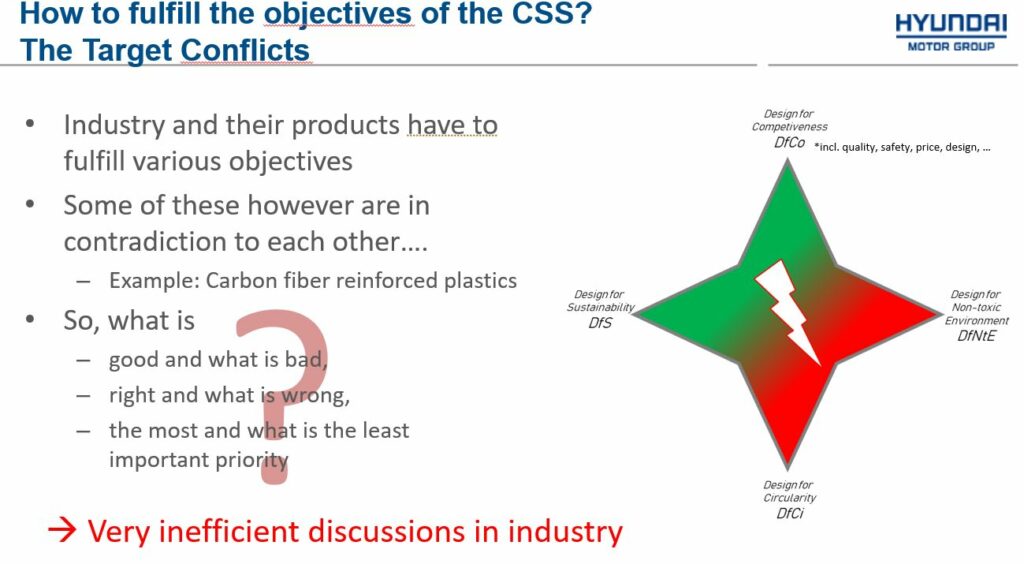
Timo Unger, Chair of the Working Group Materials & Substances, the European Automobile Manufacturers Association (ACEA). Timo raised the point about the difficulties of achieving the balance between the various challenges faced by car manufacturers. Different prioritisation for different sectors and taking a more value chain-specific approach may be a solution out of this impasse:
“If we place products on the market, we are required to design these products to be sustainable. At the same time, we are required to design them to be circular and to be non-toxic. And last but not least, our products should be of good quality and good in safety. They have to be good in price and design. Unfortunately, we really have a tough conflict here, because it’s almost impossible to fulfil all of these dimensions at the same time”
Timo Unger, Chair of the Working Group Materials & Substances, the European Automobile Manufacturers Association (ACEA)
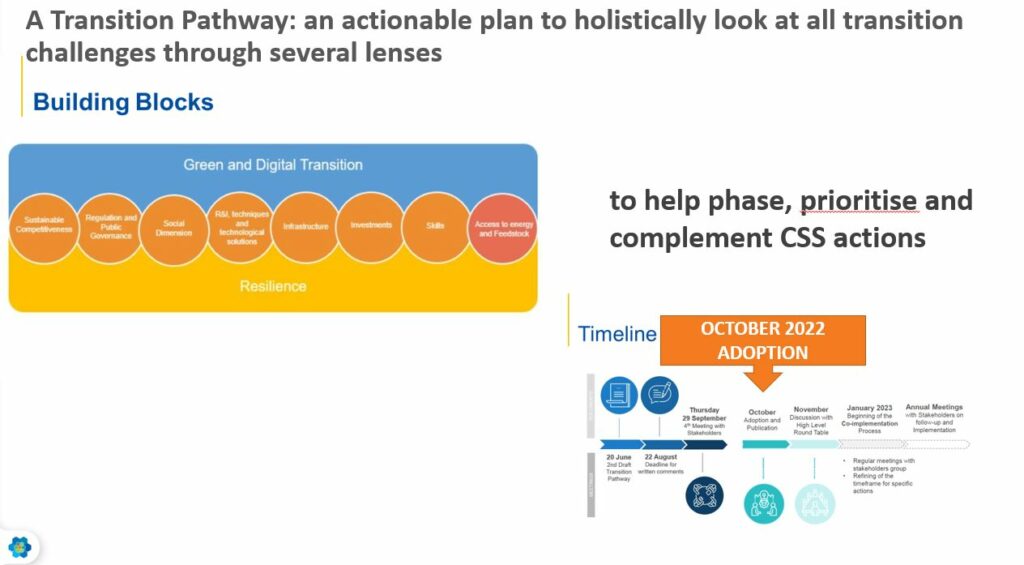
Sylvie Lemoine, Executive Director Product Stewardship at Cefic concluded the dialogue by emphasising that the chemical industry needs a Transition Pathway, an actionable plan that brings together all aspects, from regulation to the research & innovation agenda.
“We need this work plan. It will help to phase, prioritise and complement the Chemicals Strategy for Sustainability” Sylvie Lemoine, Executive Director Product Stewardship, Cefic.
Sylvie Lemoine, Executive Director Product Stewardship, Cefic